Package leaflet: Information for the Parent/Guardian
Beraksurf ® 25 mg/ml Sterile Pyrogen Free Suspension Please read this leaflet carefully before this medicine is administrated because it contains important information for you.
Sterile Intratracheal Suspension
Beraksurf® contains Beractant active substance, which is an extracted natural surfactant of bovine lungs, and is used to help respiration of premature infants.
Pharmaceutical Category
Pulmonary Surfactant
Mechanism of Action
Beraksurf® is a substitute for natural pulmonary surfactant in surfactant replacement therapy. In infants suffering from Respiratory Distress Syndrome (RDS) or in preterm infants with risk of developing RDS, this surfactant is not produced or is not present in enough amount, therefore they can’t breathe normally. Beraksurf® reduces surface tension between air and alveolar surface and prevent collapsing alveolus during respiration and thus helps infant breath normally.
Indication
Incidence of Respiratory Distress Syndrome usually is in preterm infants with age less than 32 weeks of pregnancy or in infants with birth weight between 700 to 1250 grams. In order to use as RDS prophylaxis in preterm infants or infants with evidence of surfactant deficiency, instillation of Beraksurf® should be done as soon as possible, preferably just after 15 minutes after birth.
In RDS treatment for infants need to mechanical ventilation, instillation of Beraksurf® should be done as soon as possible, preferably in 8 hours after birth.
How to use Beraksurf®
Administration method
- Before using Beraksurf® take the vial out of refrigerator and keep it in the room to reach to room temperature, or hold the vial in your hand for 8-10 minutes. Don’t use any type of heating devices for this purpose. Mix the content of vial very gently, by 3 to 5 times slow inverting the vial.
- Suspension color should be white-off creamy to light brown.
- Before use, check the vial for consistency of suspension or discoloration of its content.
- In the case of settling of content, gently invert the vial 3 to 5 times. Avoid shaking the vial content.
- According to clinician opinion, it is possible to suck the infant before instillation of Beraksurf.
- Do not dilute Beraksurf® before use.
- It is possible to see a little foaming above the content of vial.
Dosing of Beraksurf®
Needed amount of Beraksurf® for instillation is determined by specialist physician. However, normal dosing of Beraksurf is as following:
Prophylaxis Indication: 4 ml of Beraksurf® for each kilogram of infant weight at birth time (100 mg phospholipid for each kilogram of infant weight at birth time) intratracheally, as soon as possible and preferably just 15 minutes after birth.
RDS Treatment: 4 ml of Beraksurf® for each kilogram of infant weight at birth time (100 mg phospholipid for each kilogram of infant weight at birth time) intratracheally, as soon as diagnosis of RDS.
In both indications, if necessary, the mentioned dose could be repeated up to 3 times in first 48 hours of infant life. Repeating doses should not be done in less than 6 hours after the last dose. Normally, there is no need to extend time between each repeat more than 12 hours, unless Beraksurf® is deactivated by blood, meconium, or infection.
In the following table, general doses of Beraksurf® based on infant weight at birth is given
| Weight at birth (g) | Total dose (ml) |
|---|---|
| 600-650 | 2.6 |
| 651-700 | 2.8 |
| 701-750 | 3 |
| 751-800 | 3.2 |
| 801-850 | 3.4 |
| 851-900 | 3.6 |
| 901-950 | 3.8 |
| 951-1000 | 4 |
| 1001-1050 | 4.2 |
| 1051-1100 | 4.4 |
| 1101-1150 | 4.6 |
| 1151-1200 | 4.8 |
| 1201-1250 | 5 |
| 1251-1300 | 5.2 |
| 1301-1350 | 5.4 |
| 1351-1400 | 5.6 |
| 1401-1450 | 5.8 |
| 1451-1500 | 6 |
| 1501-1550 | 6.2 |
| 1551-1600 | 6.4 |
| 1601-1650 | 6.6 |
| 1651-1700 | 6.8 |
| 1701-1750 | 7 |
| 1751-1800 | 7.2 |
| 1801-1850 | 7.4 |
| 1851-1900 | 7.6 |
| 1901-1950 | 7.8 |
| 1951-2000 | 8 |
Administration Procedure
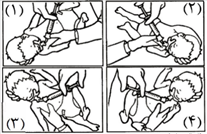
Beraksurf® is administered intratracheally by instillation through a 5 French end-hole catheter. Divide each prescribed dose into 4 aliquots and instill each part by passing the catheter through a neonatal suction valve attached to the endotracheal tube in following positions. Or instill through the catheter by briefly disconnecting the endotracheal tube from the ventilator, in the following position:
- Body and head of infant inclined 5 – 10 degrees down, head turned to the right
- Body and head of infant inclined 5 – 10 degrees down, head turned to the left
- Body and head of infant inclined 5 – 10 degrees up, head turned to the right
- Body and head of infant inclined 5 – 10 degrees up, head turned to the left
 Pharmacodynamics
Pharmacodynamics
Effect of Beraksurf® administration is improving oxygenation in some minutes after instillation.
Contraindications
There is no known contraindication for Beraksurf®.
Precautions
Beraksurf® is just for intratracheal administration. Avoid vigorous shaking of the product.
Beraksurf® can change oxygenation and pulmonary compliance very quickly, therefore its administration should be under direct supervision of specialists well experienced with intubation, ventilator management, and general care of preterm infants. Treated Infants should be frequently monitored with measurement of systemic oxygen and carbon dioxide.
If during administration, temporary bradycardia and also decrease in oxygen level occurred stop dosing procedure and stabilize the condition and then resume the dosing procedure.
Adverse Reactions
Beraksurf® like other pharmaceuticals may have some adverse reactions, although all of them could not be seen in an individual.
- Highly frequent (more than 10%): transient bradycardia
- Frequent (between 1 – 10%): Oxygen desaturation
- Non-frequent and rare (less than 1%): hypocarbia, hypercarbia, pallor, endotracheal tube reflux, vasoconstriction, endotracheal tube blockage, hypertension, hypotension, pneumothorax, apnea and post-treatment sepsis.
Storage Condition
Beraksurf® should not be used after expiration date noted on drug label. Keep Beraksurf® in refrigerator (2 – 8 degree C) and protect it from light. Keep the vial in its box until the just time of use.
Avoid freezing vial content. Frozen vials should be discarded.
Beraksurf® is a single dose medicine. After opening unused content should be discarded.
If the vial kept in room temperature more than 8 hours, it should not be returned to refrigerator again.
Packaging and Content
Each milliliter of Beraksurf® contains 25 milligrams phospholipids, neutral lipids, fatty acids like palmitic acid, surfactant proteins, sodium chloride and water for injection.
Beraksurf® is an off-white creamy to light brown sterile suspension presented in single dose vials. Each 4 ml vial contains 100 mg phospholipids and each 8 ml vial contains 200 mg phospholipids.
List of excipients
Sodium chloride 36 mg/mL, water for injection and sodium hydroxide
Beraksurf® license Holder
Tekzima Darou Alborz
Block B, No. 125, km 22.5 Tehran-Karaj Road, Alborz, Iran
Manufacturer name
Noargen Co.: Block A, No. 125, 22th Km of Tehran- Karaj Makhsous Road, Alborz, Iran
Ronak Pharmaceutical Co.: kargar Blvd, Azadegan 2 St, Kaveh Industrial Zone, Saveh, Iran.



